Introduction
Treatment for hormone-sensitive metastatic prostate cancer (mHSPC) has evolved in recent years with the addition of multiple treatment intensification options associated with androgen deprivation therapies (ADT), which were previously considered the mainstay of treatment, Treatment intensification includes taxane-containing chemotherapy and therapies that target the androgen receptor axis-directed therapies (ARATs)1.
During the past 7 years, treatment paradigms have changed dramatically with the incorporation of four treatment lines. Beginning in 2015 and continuing through 2017, docetaxel and abiraterone emerged as viable treatment options for mHSPC based on the results of pivotal trials such as CHAARTED, LATITUDE, and STAMPEDE2–5. In 2018, enzalutamide and apalutamide were added into the therapeutic armamentarium following the publication of landmark trials such as ARCHES, ENZAMET, and TITAN5–7. Recently, primary radiotherapy has become an additional treatment option for patients with oligometastatic disease, owing to insights from the STAMPEDE trial8. Many ongoing trials are testing new androgen axis inhibitors, either as monotherapies or in combination, including pioneering studies involving triplet regimens.
As newer therapies targeting the androgen receptor axis have received regulatory approval relatively recently, there is few real-world data evaluating their utilization in the mHSPC landscape; hence, the purpose of this study was to determine through a historical cohort, the characteristics, treatment patterns, and outcomes of a population with mHSPC in a referral center in Bogotá. To the best of our knowledge, this is the first local study to report these data.
Materials and methods
This was a retrospective, observational study. All patients with mHSPC who were attended at the Hospital Universitario San Ignacio, a referral hospital in Bogota, Colombia, between January 2018 and May 2022 were included in our study using convenience sampling. Our study only included metastatic disease diagnosed through conventional imaging modalities (computed tomography, bone scintigraphy, or magnetic resonance imaging).
Disease volume was defined according to the CHAARTED trial; hence, high-volume disease is when the patient had more than four osseous metastases, with at least one extra-axial, or the presence of visceral metastases, with the remainder being low volume. Metachronous disease was defined when metastatic disease occurred after an initial presentation as a localized disease, having received definitive treatment and metachronous disease was defined when metastatic disease was diagnosed at the time of the initial diagnose of mHSPC.
Standard descriptive statistics were analyzed for all variables. The results are expressed as mean or median with standard deviation or interquartile range for continuous variables, depending on whether they distributed normally, and as a number of patients with percentages for categorical data. Normal distribution was assessed with the Shapiro–Wilk test. Continuous variables were assessed through analysis of variance if normally distributed or with the Kruskal–Wallis test for non-normally distributed data and discrete variables. Categorical variables were analyzed through the chi-square test.
Survival and distribution of outcome measures were estimated using the Kaplan–Meier method. Cox proportional hazard models, stratified according to risk factors, were used to estimate hazard ratios (HR) for the time-to-event endpoints. Stratified log-rank tests were used to compare the distributions of events and times among the different groups.
All statistical calculations were performed in R (Data analysis and statistical software). A p < 0.05 was considered significant.
Results
A total of 125 patients with mHSPC were included in this study. The baseline characteristics of our population are described in table 1. Median age was of our cohort was 73.5 years (confidence interval [CI]: 71.48-75.31), the median PSA was 209 ng/mL, and 90% of patients had synchronous mHSPC. The distribution of high-volume mHSPC was 92% and that of M1b was 91%. Of these patients, 21% underwent surgical castration and 79% received pharmacological treatment.
Table 1. Baseline demographic and clinical characteristics
| Sample characteristics (n = 125) | |||
|---|---|---|---|
| Variable | n | % | p-value |
| Age (years) mean = 73.4 SD (10.9) CI (71.489-75.311) | 0.56 | ||
| ECOG | |||
| 0-1 | 66 | 52.80 | 0.28 |
| 2-4 | 59 | 47.20 | |
| PSA (ng/ml) mean = 529.6 CI (349.913-709.287) | 0.07 | ||
| Type of castration | |||
| Pharmacological | 95 | 76.00 | 0.002 |
| Orchiectomy | 27 | 21.60 | |
| PSA (ng/mL) 3 months mean = 54.7 CI (37.871-71.529) | |||
| PSA (ng/mL) 6 months mean = 23.4 CI (12.882-33.918) | |||
| Grade group gleason (GGG) | |||
| 1 | 8 | 6.40 | 0.02 |
| 2 | 9 | 7.20 | |
| 3 | 0 | 0.00 | |
| 4-5 | 58 | 46.40 | |
| cT | |||
| ≤ T2 | 30 | 24.00 | 0.86 |
| > T2 | 70 | 56.00 | |
| Tx | 25 | 20.00 | |
| cN | |||
| N1 | 39 | 31.20 | 0.07 |
| N0 | 58 | 46.40 | |
| cM | |||
| M1a | 9 | 7.20 | 0.000016 |
| M1b | 91 | 72.80 | |
| M1c | 25 | 20.00 | |
| Disease volume | |||
| Low volume | 33 | 26.40 | < 0.00001 |
| High volume | 92 | 73.60 | |
| Therapy | |||
| ADT | 50 | 40.00 | 0.096 |
| Taxane + ADT | 37 | 29.60 | |
| ARAT + ADT | 35 | 28.00 | |
| Other | 1 | 0.80 | |
| Therapy discontinuation | |||
| Yes | 12 | 9.60 | < 0.00001 |
| No | 113 | 90.40 | |
| Local disease treatment | |||
| Radical prostatectomy ± Lymphadenectomy | 6 | 4.80 | 0.000116 |
| Radiotherapy | 10 | 8.00 | |
| None | 109 | 87.20 | |
| Timing | |||
| Synchronic | 85 | 68.00 | 0.004 |
| Metachronous | 40 | 32.00 | |
|
CI: confidence interval; SD: standard deviation; ARAT: androgen receptor axis-directed therapies; ADT: androgen deprivation therapies. |
|||
Table 2 and figure 1 show the changes in the percentage of patients within each treatment regimen. In 2018, 41% of patients received ADT exclusively, 30% were treated with taxanes, and 28% received ARATs. Overtime the percentage of patients receiving ADT exclusively decreased going from 41% by 2019 to 15% by 2022.
Table 2. Therapy distribution trends
| Year | Therapy | n | % |
|---|---|---|---|
| 2018 | ADT | 1 | 100 |
| Taxane | 0 | 0 | |
| ARAT | 0 | 0 | |
| Other | 0 | 0 | |
| 2019 | ADT | 14 | 56.00 |
| Taxane | 7 | 28.00 | |
| ARAT | 4 | 16.00 | |
| Other | 0 | 0.00 | |
| 2020 | ADT | 18 | 43.90 |
| Taxane | 12 | 29.27 | |
| ARAT | 11 | 26.83 | |
| Other | 0 | 0.00 | |
| 2021 | ADT | 16 | 30.77 |
| Taxane | 17 | 32.69 | |
| ARAT | 18 | 34.62 | |
| Other | 1 | 1.92 | |
| 2022 | ADT | 1 | 14.29 |
| Taxane | 0 | 0.00 | |
| ARAT | 6 | 85.71 | |
| Other | 0 | 0.00 | |
|
ARAT: androgen receptor axis-directed therapies; ADT: androgen deprivation therapies. |
|||
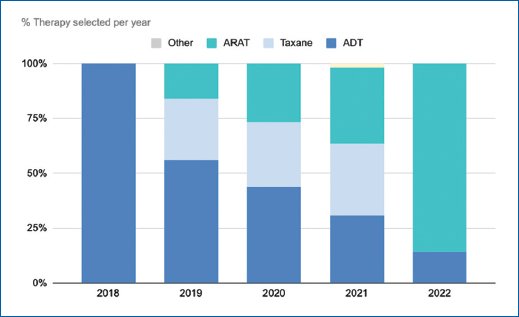
Figure 1. Selected therapy per year.
Analysis of the baseline characteristics of our population through the type of therapy received is shown in table 3. Patients who received ADT exclusively were older and had a lower disease volume. Those receiving ADT and ARAT had a lower median PSA compared to patients receiving taxane intensification therapy.
Table 3. Epidemiologic factors and associations
| Variable | ADT (n = 50) | 0 (%) | Taxane (n = 37) | 37 (%) | ARAT (n = 35) | 35 (%) | p-value | Two groups comparison P value |
|---|---|---|---|---|---|---|---|---|
| Age (mean, IC) | 78.3 (74.946-81.654) | 69.2 (67.00-71.391) | 70.7 (67.321-74.079) | 0.56 | ARAT versus Taxane P = 0.127
ARAT versus ADT P = 0.006 Taxane versus ADT P ≤ 0.00001 |
|||
| ECOG | ||||||||
| 0-1 | 44 | 88 | 33 | 89 | 32 | 91 | 0.23 | ARAT versus Taxane P = 0.15
ARAT vs ADT P = 0.15 Taxane versus ADT P = 0.92 |
| 2-4 | 6 | 12 | 3 | 8 | 3 | 9 | ||
| PSA (ng/mL) | 309 (164.889-453.711) | 656 (243.241-1,068.7) | 4067 (2,067-10,302) | 0.3 | ||||
| Type of castration | ||||||||
| Pharmacological | 39 | 78 | 23 | 62 | 26 | 74 | 0.15 | |
| Orchiectomy | 8 | 16 | 11 | 30 | 8 | 23 | ||
| PSA (ng/mL) 3 months | 38.8 (19.92-57.676) | 80.9 (39.249-122.551) | 42.2 (20.866-63.9) | 0.12 | ||||
| PSA (ng/mL) 6 months | 15.2 (6.635-23.765) | 35.4 (8.374-62.4) | 16.5 (3.248-29.7) | 0.001 | ARAT versus Taxane = 0.00092
ARAT vs ADT P = 0.32 Taxane versus ADT P = 0.01 |
|||
| Grade group Gleason (GGG) mean | 4 | 4 | 4 | |||||
| cT | ||||||||
| ≤ T2 | 13 | 26 | 23 | 62 | 6 | 17 | 0.99 | |
| > T2 | 26 | 52 | 7 | 19 | 10 | 29 | ||
| Tx | 12 | 24 | 7 | 19 | 19 | 54 | ||
| cN | ||||||||
| N1 | 21 | 57 | 14 | 67 | 17 | 63 | 0.49 | |
| N0 | 18 | 49 | 7 | 33 | 10 | 37 | ||
| cM | ||||||||
| M1a | 4 | 8 | 0 | 0 | 5 | 14 | 0.44 | |
| M1b | 36 | 72 | 29 | 78 | 24 | 69 | ||
| M1c | 10 | 26 | 8 | 22 | 6 | 17 | ||
| Brain metastasis | 0 | 0 | 0 | 0 | 0 | 0 | ||
| Hepatic metastasis | 2 | 4 | 4 | 11 | 2 | 6 | ||
| Lung metastasis | 5 | 10 | 7 | 19 | 4 | 11 | ||
| Metastatic disease volume | ||||||||
| Low volume | 21 | 42 | 0 | 0 | 12 | 34 | 0.002 | ARAT versus Taxane = 0.013
ARAT versus ADT P = 0.49 Taxane versus ADT P = 0.00093 |
| High volume | 29 | 58 | 37 | 100 | 23 | 66 | ||
| Therapy discontinuation | ||||||||
| Yes | 2 | 4 | 6 | 6 | 3 | 9 | 0.53 | |
| No | 39 | 78 | 28 | 76 | 30 | 86 | ||
| Localized disease treatment | ||||||||
| Radical prostatectomy ± Lymphadenectomy | 4 | 8 | 1 | 3 | 1 | 3 | 0.39 | |
| RT | 5 | 10 | 0 | 0 | 5 | 14 | ||
| None | 45 | 90 | 36 | 97 | 30 | 86 | ||
| Temporality | ||||||||
| Synchronous | 29 | 58 | 28 | 76 | 26 | 74 | 0.89 | |
| Metachronous | 4 | 8 | 1 | 3 | 3 | 9 | ||
| % overall mortality | 10 | 20 | 5 | 14 | 2 | 6 | 0.64 | |
| Overall survival (months) OS | 40 | 80 | 32 | 86 | 33 | 94 | ||
|
ARAT: androgen receptor axis-directed therapies; ADT: androgen deprivation therapies. |
||||||||
The median follow-up time was 9 months, biochemical recurrence-free survival rate was 78%, and overall survival rate was 86% (Fig. 2). The analysis of progression-free survival and time to castration resistance was significantly influenced by varying follow-up durations between the introduction of different therapies. The average time to castration resistance in patients receiving exclusive ADT was 18 months, with a 95% CI of 13.676-22.324. Calculating the time to castration resistance in other therapies is constrained due to the mean follow-up, resulting in biased estimations.
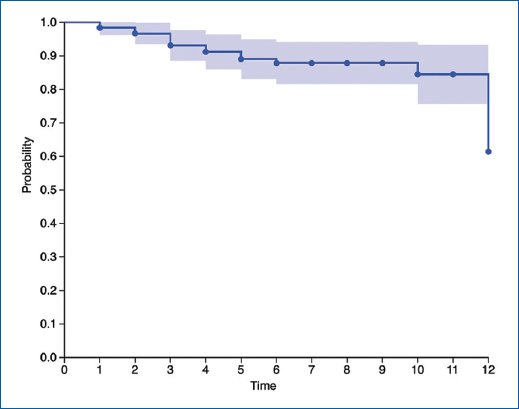
Figure 2. Survival analysis. Kaplan–Meier estimates of OS in all patients.
Discussion
To the best of our knowledge, this is the first local study to publish the treatment patterns of patients with mHSPC and represents real-world evidence on the current treatment approach for patients with this condition.
Our study had several noteworthy findings, such as the increase in the usage of intensification regimens. This upward trend can be attributed to the heightened awareness among physicians regarding the efficacy of these innovative therapeutic approaches. This heightened awareness is, in turn, substantiated by a body of research that has consistently demonstrated superior survival outcomes associated with such regimens, which we could not demonstrate due to a short period of follow-up.
Three studies justify the use of chemotherapy in combination with ADT. All trials compared ADT alone as the standard treatment with ADT combined with immediate docetaxel (75 mg/m2, every 3 weeks within 3 months of starting ADT); the main outcome was overall survival. The follow-up time was between 29 and 50 months. The studies independently demonstrated an improvement in the primary outcome with the addition of docetaxel. The meta-analysis of these studies showed an increase in overall survival by adding chemotherapy to ADT (HR: 0.77; 95% CI: 0.68-0.87; p < 0.0001)9.
Representative studies on the use of abiraterone and ADT include the STAMPEDE and LATITUDE trials. The regimen was abiraterone acetate (1000 mg daily) plus prednisone (5 mg daily) during ADT in men with mHSPC. The main outcome measure was also overall survival. The two studies demonstrated an impact on overall survival for the combination in a follow-up time between 30 and 40 months3,4.
There are two large clinical trials on the use of enzalutamide in combination with ADT, ENZAMET, and ARCHES. In ARCHES, the primary endpoint was radiological progression-free survival (rPFS), finding a benefit with an HR of 0.39 (0.3-0.5). In ENZAMET as the primary outcome, overall survival was assessed with a follow-up period of 34 months, revealing a statistically significant difference, with a HR of 0.67 (95% CI: 0.52-0.86)5,6.
In the TITAN trial, apalutamide was used as an ARAT, with rPFS and overall survival as coprimary outcomes. rPFS with a HR of 0.48 (0.39-0.6) and overall survival at 24 months improved with the combination with a HR of 0.67 (0.51-0.89)7.
Findings of our study are similar with those of other real-world evidence studies, where there is still a high percentage of patients exclusively receiving ADT, for example, in their study, Karim et al.10 which carried put his study in Alberta, Canada, with data from January 2016 to 31 December 2020, also found an increase in the usage of intensification regimens, that patients who received ADT exclusively were older and had a lower PSA compared to those treated with intensification therapies. Leith et al. who carried out the largest real-world evidence study which included data from seven countries also found an increase in the usage of intensification regimens11.
It should be noted that there is an important proportion of patient that still receives ADT exclusively as their main therapy; in some patients, this is driven by their physical status, compliance, tolerance to adverse events, and the balance of impact on quality of life versus overall survival, which is an important factor when choosing or not an intensification therapy as showed in other real-world evidence studies. The reasons for exclusive ADT were listed in relation to poor functional status, compliance problems, and probability of adverse events. These results were consistent with those of our cohort11,12.
Our study has some limitations, first, this is a retrospective study carried out in only one health institution, and second all data was collected from previous medical records, which may not include all of the relevant information or could contain mistakes that could have led to misinterpretation.
Conclusion
A description of the population of a national reference center for the treatment of hormone-sensitive prostate cancer was made included its demographic characteristics according to global trends. This is the first study of its kind in the local context.
Funding
The authors declare that they have not received funding.
Conflicts of interest
The authors declare no conflicts of interest.
Availability of data and material
The authors declare data transparency.
Ethical disclosures
Protection of human and animal subjects. The authors declare that no experiments were performed on humans or animals for this study.
Confidentiality of data. The authors declare that they have followed the protocols of their work center on the publication of patient data.
Right to privacy and informed consent. The authors have obtained approval from the Ethics Committee for analysis and publication of routinely acquired clinical data and informed consent was not required for this retrospective observational study.
Use of artificial intelligence for generating text. The authors declare that they have not used any type of generative artificial intelligence for the writing of this manuscript nor for the creation of images, graphics, tables, or their corresponding captions.
Ethics
This study is classified as non-risk not necessary to request informed consent; also an institutional review board number was not required due to the observational and retrospective nature of the study.