Introduction
Periodontal disease (PD) is a chronic disease caused by inflammation related to infection, which has a negative impact on the tooth’s supporting structures1. The main etiology of PD is aggressive disorganized biofilm and periodontal microorganisms2. At present, PD remains a global public health issue, as evidenced by 1.09 billion cases and 91.5 million new cases recorded in 2019 globally3. PD disrupts the chewing process, lowers quality of life, reduces self-confidence, and affects dental esthetics4–6. Moreover, PD has been linked to various chronic diseases and systemic conditions7–12, including cancer13.
Prostate cancer (PC) is a type of non-skin cancer which is caused by chronic inflammation and infection of the prostate gland14. With about 1.4 million new cases and over 365,000 deaths annually, PC is one of the most prevalent malignancies in the world15. Risk factors for PC include tobacco/cigarettes use, diet, physical activity, metabolic syndrome, alcohol intake, and specific medications16–19.
Evidence suggests that PD is linked to the development of PC. An imbalance of oral pathogens has been linked to infection and inflammation of the prostate gland20. This evidence is corroborated by a study of the relationship between oral microbiome and PC, where the relationship is believed to be due to chronic inflammation, periodontal pathogens, periodontal inflammatory mediators and their expression, and the induction of inflammatory cytokines in neoplastic tissue21. In addition, PD and PC shared several risk factors, including smoking, drug use, age, genetic predisposition, and diet16,22,23.
Exploration of the link between PD and PC showed inconsistent conclusions. Some studies showed a significant24–27, whereas others showed no significant association28–32. In addition, two meta-analyses conducted in the previous 5 years did conclude that PD was associated with an increased risk of PC33,34; however, one of these meta-analyses included studies with ineligible comparisons, which could have been highly biased. In addition, meta-analysis needs to be reconducted to update the studies published afterward. Therefore, this study aims to determine the relationship between PD and PC using a systematic review with meta-analysis.
Material and methods
Protocol and registration
This study is a systematic review and meta-analysis following the PRISMA 2020 guidelines35. The study protocol was registered in PROSPERO with registration number CRD420250654902 (available on www.crd.york.ac.uk/).
Focused question
The research question in this study was “Is there an association between PD and PC?” To address the research question, the population, exposure, comparison, outcomes, and study framework were applied, detailed in table 1.
Table 1. PECOS framework
| Element | Details |
|---|---|
| Population | Male population |
| Exposure | PD |
| Comparison | Men without PD or healthy controls |
| Outcomes | PC |
| Study | Cohort, case-control, cross-sectional studies |
|
PD: periodontal disease; PC: prostate cancer; PECOS: population, exposure, comparison, outcomes, and study. |
|
Search strategy
A systematic and comprehensive literature was conducted in PubMed, Scopus, ScienceDirect, and Google Scholar from inception to 2024. Terms applied in literature search are as follows: (“periodontal” [All Fields] OR “periodontally” [All Fields] OR “periodontically” [All Fields] OR “periodontics” [MeSH Terms] OR “periodontics” [All Fields] OR “periodontic” [All Fields] OR “periodontitis” [MeSH Terms] OR “periodontitis” [All Fields] OR “periodontitides” [All Fields]) AND (“prostatic neoplasms” [MeSH Terms] OR (“prostatic” [All Fields] AND “neoplasms” [All Fields]) OR “prostatic neoplasms” [All Fields] OR (“prostate” [All Fields] AND “cancer” [All Fields]) OR “ prostate cancer” [All Fields]).
Eligibility criteria
Studies were included if they satisfied the subsequent requirements for inclusion: (1) any observational studies (cohort, case–control, or cross-sectional studies), (2) research subjects using two groups: PD group and non-PD group or healthy periodontal condition group, (3) articles reporting odds ratio (OR), relative risk (RR), or hazard ratio (HR) with 95% confidence interval (CI), and (4) peer-reviewed full-text articles. Conversely, studies were excluded if they met the exclusion criteria, including review articles, editorials, experimental articles, commentaries, case reports, and case series. For two or more studies using the same dataset, if any, we selected and included the study involving larger participants and/or having more rigorous and comprehensive methods. Other restraints, including publication year and language, were not applied. Therefore, all articles published from inception to 2024 were taken into consideration for inclusion, which provided that they satisfied the inclusion criteria.
Quality assessment
Study quality was assessed using the Newcastle-Ottawa Scale (NOS)36. NOS has three assessment categories, namely, selection, comparability, and outcome with maximum scores of four, two, and three, respectively. The NOS assessment has a total score range of 0-9, with ≥ 7 denoting high quality, 4-6 denoting moderate quality, and ≤ 4 denoting low quality. Two reviewers (FMR and RA) were assigned to assess the study quality and validated by the senior researcher (JI). Any discrepancies between reviewers in the study quality assessment process were resolved through careful discussion and decision-making.
Data extraction
Two authors (R.A. and S.M.C.) independently read all included articles and extracted data. Table was used to extract data from each included study, consisting of authors/references, country, study design, sample size, age, PD assessment, PC assessment, adjustments for confounding factors, and estimates in the form of OR, RR, or HR along with 95% CI. Furthermore, all study data were comprehensively discussed quantitatively and qualitatively in the discussion.
Statistical analysis
For all statistical analyses, Stata version 17.0 software (StataCorp LLC, College Station, Texas, USA) was used. The findings in this meta-analysis were reported with forest plots and presented as pooled OR with 95% CI to measure the association between PD and PC. RR and HR were treated as OR when combined in this meta-analysis. We performed the study heterogeneity analysis (I2) with the stipulation of low and high heterogeneity if I2 was ≤ 50% (or p ≥ 0.1) and > 50% (or p < 0.1), respectively. If study heterogeneity was low, a fixed-effects model meta-analysis was used, whereas a random-effects model meta-analysis was applied if study heterogeneity was high37. Subgroup analyses were also performed stratified by country region, study design, sample size (< 10.000 and ≥ 10.000), and follow-up period (< 15 and ≥ 15 years). Funnel plot was not used in this study, in accordance with the provision that funnel plot is used if the number of included studies is more than 10 studies38; however, publication bias analysis was performed using Egger’s test39 and Begg and Mazumdar’s test40. Sensitivity analysis was conducted to analyze the robustness of the meta-analysis, using the leave-one-out method by omitting the studies one by one and then re-analyzing the remaining studies41.
Results
Study selection
The literature search in electronic databases yielded, after any duplicates were removed, 1,159 records. After initial screening, 676 papers were excluded, 483 articles remain. Four hundred and fifty-three reports were not retrieved based on screening on titles and abstracts; 30 articles remain. The remaining articles were screened for eligibility, with 23 articles excluded due to irrelevant objectives, review articles, method issues, ineligible comparisons, and covering the same dataset. In addition, we also conducted a literature search through other sources, including citation searches, and obtained two records that met the inclusion criteria. Nine studies were finally included in this meta-analysis. The study selection process is depicted in figure 1.
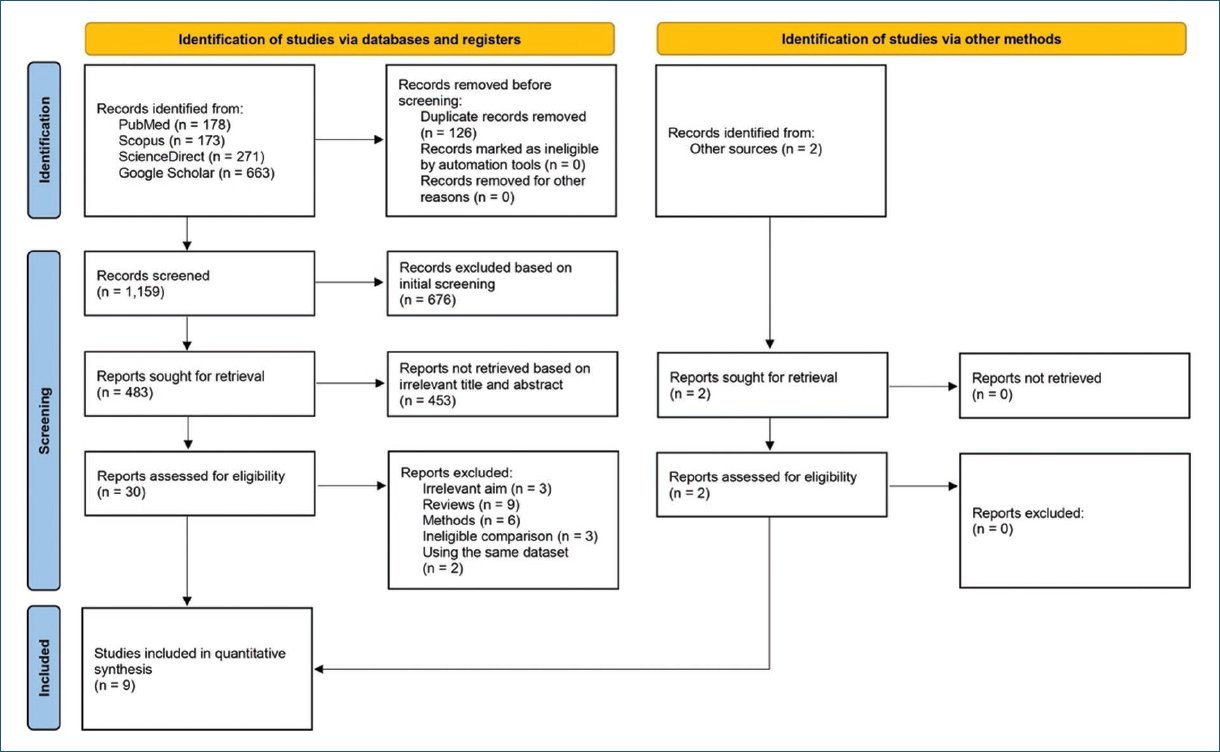
Figure 1. PRISMA flowchart.
Characteristics of included studies
This meta-analysis involved nine cohort studies with a total of 621,982 male participants in several regions in Asia including Taiwan25,26 and South Korea27, Europe including Sweden24,30 and Finland28, and North America including the USA29,31,32. Based on the study design, six articles were prospective cohort studies and the remaining three were retrospective cohort studies. Table 2 describes the characteristics of every study that was included.
Table 2. Characteristics of included studies
| Reference | Country | Study design | Sample size (n) | Age (years) | Follow-up (years) | Periodontal disease assessment | Prostate cancer assessment | Adjustments | Estimates (95% CI) |
|---|---|---|---|---|---|---|---|---|---|
| Arora et al.24 | Sweden | Prospective cohort | 6,961 | 38-77 | 27 | Self-report | ICD | Age, education, employment, number of siblings, smoking status, smoking status of partner, alcohol intake, body mass index, and diabetes | HR 1.47 (1.04-2.07) |
| Chen et al.25 | Taiwan | Prospective cohort | 327,046 | ≥ 20 | 11.8 | ICD-9 code 523.3 and 523.4 or ICD-10 code K05.2 and K05.3 | ICD-9 code 185 or ICD-10 code C61 | Age, Charlson comorbidity index score, and NSAID use | HR 1.22 (1.10-1.35) |
| Chung and Chan26 | Taiwan | Retrospective cohort | 43,052 | > 65 | 8.2 | Oral examination | ICD-9 or ICD-10 | Age, education, marital status, and smoking status | HR 1.340 (1.019-1.762) |
| Heikkilä et al.28 | Finland | Retrospective cohort | 28,180 | ≥ 29 | 10 | Medical records | ICD-O-3 C61 | Calendar time, age, and socio-economic status | RR 1.75 (0.63-4.87) |
| Hujoel et al.29 | USA | Prospective cohort | 4,466 | 25-74 | 10 | ICD-9 | ICD-9-185.0 | Age | OR 1.81 (0.76-4.34) |
| Lee et al.27 | South Korea | Retrospective cohort | 187,934 | ≥ 40 | 12 | KCD-6 codes K05.2-K05.6 | KCD-6 codes C61 | Age, household income, insurance status, hypertension, diabetes, cerebral infarction, angina pectoris, myocardial infarction, smoking status, alcohol intake, and regular exercise | HR 1.14 (1.01-1.31) |
| Meurman et al.30 | Sweden | Prospective cohort | 1,032 | 62-72 | 30 | ICD-10 periodontal diagnostic codes | ICD-10 code C61 | Age, income, and education | OR 1.734 (0.932-3.227) |
| Michaud et al.31 | USA | Prospective cohort | 19,933 | 40-75 | 26 | Self-report | Medical records | Age, race, alcohol intake, physical activity, diabetes, body mass index, geographical location, height, and NSAID use | HR 1.17 (0.94-1.47) |
| Michaud et al.32 | USA | Prospective cohort | 3,378 | 44-66 | 14.7 | CDC-AAP | Medical records | Age, field center, education, smoking status and duration, alcohol intake, body mass index, and diabetes | HR 1.01 (0.82-1.23) |
|
AAP: American Academy of Periodontology; CDC: Centers for Disease Control and Prevention; HR: hazard ratio; ICD: International Classification of Diseases; KCD: Korean Standard Classification of Diseases; NSAID: non-steroidal anti-inflammatory drugs; OR: odds ratio; RR: relative risk. |
|||||||||
Quality assessment of included studies
The results of quality assessment showed that three studies25,28,30 received an overall score of 9, two studies27,32 received an overall score of 8, and four studies24,26,29,31 were assessed with a total score of 7. In conclusion, all studies included in this meta-analysis were of high quality.
Association between PD and PC
The results of a fixed-effects model meta-analysis showed that PD was significantly associated with PC (OR = 1.19; 95% CI = 1.12-1.28; p = 0.0000), with heterogeneity test showing low heterogeneity (p = 0.42; I2 = 2.33%), (Fig. 2).
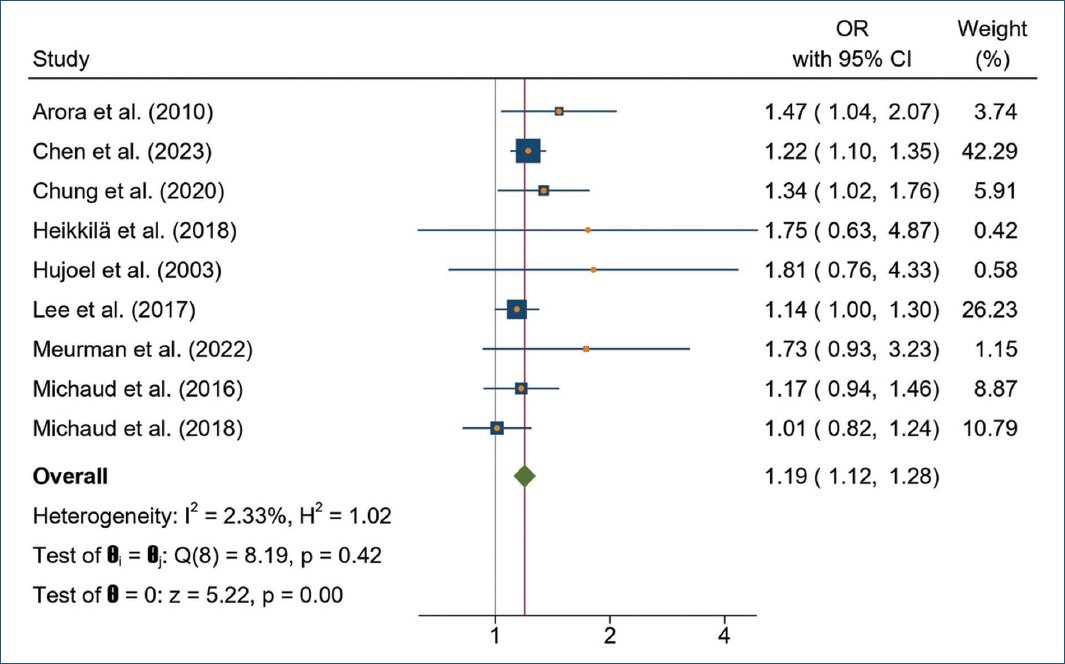
Figure 2. Forest plot of the association between periodontal disease and prostate cancer.
Subgroup analysis of the association between PD and PC
Subgroup meta-analyses stratified by country region, study design, sample size, and follow-up period were performed. All subgroup meta-analyses are presented in table 3.
Table 3. Subgroup analysis of the association between PD and PC
| Subgroup | Studies (n) | OR (95% CI) | p | Heterogeneity | |
|---|---|---|---|---|---|
| p | I2 (%) | ||||
| Overall | 9 | 1.19 (1.12-1.28) | 0.0000 | 0.42 | 2.33 |
| Country Asia Europe North America | 333 | 1.20 (1.11-1.30)1.55 (1.16-2.06)1.10 (0.95-1.27) | 0.00000.0030.23 | 0.520.870.33 | 0.000.0010.16 |
| Study design Prospective cohort Retrospective cohort | 63 | 1.20 (1.11-1.30)1.18 (1.05-1.33) | 0.00000.005 | 0.260.43 | 22.680.00 |
| Sample size < 10,000 ≥ 10,000 | 45 | 1.32 (0.97-1.79)1.20 (1.12-1.29) | 0.080.0000 | 0.100.75 | 52.630.00 |
| Follow-up period < 15 ≥ 15 | 63 | 1.18 (1.10-1.27)1.29 (1.08-1.54) | 0.00000.006 | 0.380.34 | 5.037.27 |
|
PD: periodontal disease; PC: prostate cancer, OR: odds ratio; CI: confidence interval. |
|||||
Based on a meta-analysis stratified by country regions, PD was significantly associated with PC in Asian (OR = 1.20; 95% CI = 1.11-1.30) and European populations (OR = 1.55; 95% CI = 1.16-2.06), but not significantly in North American populations (OR = 1.10; 95% CI = 0.95-1.27). PD and PC were observed to be significantly associated based on both prospective (OR = 1.20; 95% CI = 1.11-1.30) and retrospective cohort study designs (OR = 1.18; 95% CI = 1.05-1.33). In terms of sample size, the association between PD and PC was considered significant in studies with sample sizes of more than 10,000 men (OR = 1.20; 95% CI = 1.12-1.29); conversely, it was not significant in studies with sample sizes of < 10,000 men (OR = 1.32; 95% CI = 0.97-1.79). Subgroup analysis stratified by follow-up period showed a significant association between PD and PC both in follow-up periods < 15 years (OR = 1.18; 95% CI = 1.10-1.27) and ≥ 15 years (OR = 1.29; 95% CI = 1.08-1.54).
Sensitivity analysis
The leave-one-out method was used for the sensitivity analysis of this meta-analysis. The results of removing one study at a time showed no significant changes in the pooled OR and 95% CI or p-value (Fig. 3). The results of this sensitivity analysis reveal that the results of this meta-analysis are robust.
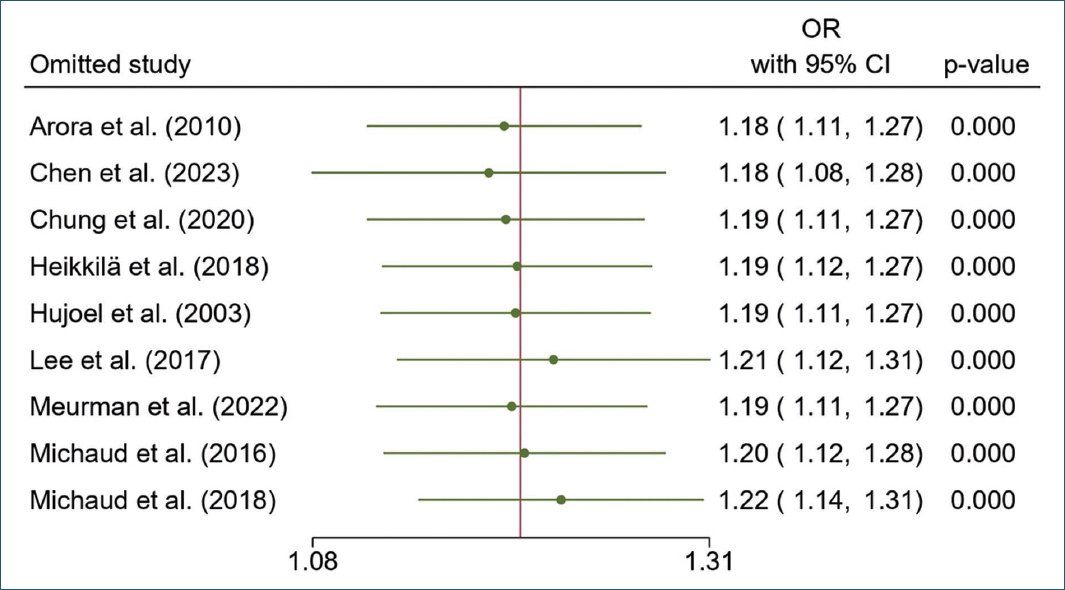
Figure 3. Leave-one-out analysis of sensitivity.
Publication bias
There was no publication bias observed across the studies, according to the results of the Egger’s test (p = 0.1025) and the Begg and Mazumdar’s test (p = 0.1753).
Discussion
The present meta-analysis, which included nine cohort studies with a total of 621,982 male participants, found that PD had a significant association with PC. Subgroup meta-analysis stratified by study design and follow-up period resulted in consistent and significant results, but non-significant results were found when stratified by country and sample size. However, this meta-analysis is robust based on the results of sensitivity analysis, and no publication bias was observed based on the results of the Egger’s test and the Begg and Mazumdar’s test.
A previous meta-analysis was conducted by Wei et al.34 in 2020 which included seven studies with 293,904 participants. Around the same year, a similar meta-analysis was also conducted by Guo et al.33 involving 440,911 participants from nine studies. However, their meta-analysis included three studies42–44 that were excluded in our meta-analysis due to the ineligibility of the comparisons, where two studies42,43 did not have a periodontally healthy control group and one44 used a comparison of gingivitis patients. Therefore, with the addition of new studies with a total of 621,982 participants and the tightening of study selection, our meta-analysis is the most recent and most comprehensive to date.
To date, the mechanisms explaining the link between PD and PC have not been clearly mapped, but chronic inflammation and periodontal pathogens invasion are believed to be linked to the risk of PC. It is caused by the spread of bacteria and/or bacterial toxins from the oral cavity or periodontal tissue through the bloodstream, causing chronic systemic inflammation. This condition, in turn, increases the risk of initiating neoplastic transformation45,46. Other evidence suggests that migration of certain periodontal pathogens, Porphyromonas gingivalis, Treponema denticola, Fusobacterium nucleatum, and Actinobacillus actinomycetemcomitans, to the prostate gland contributes to prostatic inflammation and the development of PC21.
P. gingivalis, one of the most common periodontal pathogens, has carcinogenic properties, where the pathogen inhibits apoptosis, increases cellular mitosis and proliferation, decreases adaptation to oxidative stress, induces cyclooxygenase-2, and induces immune pathways such as Toll-like receptor, causing production of inflammatory mediators such as tumor necrosis factor-α, interleukin (IL)-6, IL-8, IL-1β, prostaglandin E2, and matrix metalloproteinases47–49. Further evidence reveals that programmed cell death ligand 1 (PD-L1) expression on PC cells is increased after P. gingivalis infection50, where high PD-L1 expression has been associated with proliferation, aggressiveness, and as an unfavorable prognostic indicator in PC patients51. Lipopolysaccharide from P. gingivalis also activates the AKT pathway through IL-6/IL-6Rα/glycoprotein 130, triggering unbalanced proliferation and apoptosis of prostate cells49. In conclusion, evidence suggests that periodontal pathogens contribute to systemic chronic inflammation that further increases the risk of developing PC. However, the exact mechanism of the association between PD and PC needs to be proven through future studies.
There are several strengths to our meta-analysis. First, this is an update and improvement of previous meta-analyses, making this study the most recent and more comprehensive to date. Second, study selection was conducted comprehensively, without any restraint on the year of publication or language, and was quite serious in addressing, especially, cohort studies that are prone to using the same dataset. Third, the included studies exhibited high quality according to two reviewers’ quality evaluation results and senior researcher validation. Fourth, this meta-analysis has low heterogeneity, no publication bias, and robust sensitivity analysis results. Fifth, the estimates used in each study are estimates that have been adjusted for confounding factors.
Nonetheless, this meta-analysis acknowledges several limitations. First, controversy in the assessment of PD remains, with two studies24,31 using self-report to categorize PD participants, one study28 using medical records, and six studies25–27,29,30,32 using standard oral examination and/or clinical diagnosis. These conceptual differences may affect the final results. Second, no subgroup analysis stratified by the severity of PD was conducted, because almost all studies did not analyze the association between PD severity and PC. These two limitations are our concern, where the establishment of PD diagnosis for future studies may be warranted using the latest criteria to make the results more valid52 and analysis based on the PD severity on PC could be conducted to evaluate their link based on the PD severity. Finally, future studies with robust methods regarding the criteria and severity of PD may address the limitations of this study.
Conclusion
This meta-analysis shows a significant association between PD and PC. Chronic inflammation and migration of periodontal pathogens to the prostate gland are thought to be one of the mechanisms of PC development. Future studies may be conducted using larger sample size, more rigorous methods in establishing the diagnosis of PD and PC, and exploring the impact of periodontal treatment interventions on PC development.
Funding
The authors declare that this work was carried out with the authors’ own resources.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical considerations
Protection of humans and animals. The authors declare that no experiments involving humans or animals were conducted for this research.
Confidentiality, informed consent, and ethical approval. The study does not involve patient personal data nor requires ethical approval. The SAGER guidelines do not apply.
Declaration on the use of artificial intelligence. The authors declare that no generative artificial intelligence was used in the writing of this manuscript.
