Introduction
Genitourinary birth defects are reported to be present in approximately 10-30% of all newborns and can lead to varying degrees of morbimortality1,2. Congenital kidney anomalies are responsible for up to 50% of chronic renal failure in children worldwide2. A cohort study conducted by Calderon-Margalit et al.3 found a hazard ratio of 4.19 (95% confidence interval 3.52-4.99) for end-stage renal disease (ESRD) in patients with a history of childhood kidney disease after 30 years of follow-up. Mild kidney abnormalities in childhood could be a risk factor for ESRD in adulthood, even if there is no compromise of renal function at the time of diagnosis3. Similarly, in a study that followed a cohort of children who survived Wilms tumor, Chu et al.4 found abnormal ambulatory blood pressure monitoring in 76%, masked hypertension in 34% and microalbuminuria in 6% of participants. Timely treatments may reduce complications that otherwise would contribute to increased infant morbidity and mortality with lifelong permanent disabilities5. On the other hand, congenital anomalies that affect genitals have a lifelong impact on multiple domains, including urinary function, sexuality, fertility, and psychosocial wellness6. In both scenarios, identification and timely management of cases are essential to positively impact prognosis.
According to Global Health Estimates7, congenital anomalies were in the top 10 global causes of disability-adjusted life years (DALYs) in 2019. Although the burden of these conditions dropped worldwide from 61.815 million DALYs in 2010-51.797 million DALYs in 20198, disparities between regions persist. Around 94% of congenital anomalies occur in low- and middle-income countries9. The large proportion of cases coupled with low coverage of surgical care in these settings delay the access to timely treatment, which in turn increases the burden of disease. Delays related to presentation to medical care, referral for specialized care and surgery have been demonstrated be longest in low-income countries (e.g., Kenya) compared to high-income countries (e.g., Canada)10.
Spatial analysis has emerged as a practical tool for epidemiological research since it allows the detection of spatial disparities in the occurrence of diseases11,12. A spatial cluster is an unusual number of cases within a population in a period13. They can reveal trends that may not be apparent at an inpidual level, therefore, are useful for health services planning14. Understanding the distribution of health access is essential for the design of health interventions and resources distribution. The aim of the present study is to evaluate geographical distribution in the health-care access to subspecialized medical care of patients with genitourinary birth defects in Colombia.
Materials and methods
Data collection
Colombia is pided into 32 departments and 1119 municipalities. According to the 2018 national census, the 77.1% of population lives in urban areas and approximately 25% resides in four main cities (Bogotá, Medellín, Cali, and Barranquilla)15. The largest proportion of the population (70.1%) is located where the high complexity/specialized university hospital are in the central region, followed by 21.9% inhabit the north region, the 3% in the east, 2.7% in the west, and 2.4% in the south16.
The Inpidual health records system (Registros Inpiduales de Prestación de Servicios de Salud-RIPS, by its Spanish acronym) is a data repository for the management, regulation, and control processes for the health services required by the General System of Social Security in Colombia17. Their aim is to follow-up on the health services provided, evaluate service coverage, formulate health policies as well as allocate financial and human resources. The information must be organized according to guidelines proposed by the Ministry of Health. Data registration is mandatory and performed monthly by health-providing institutions and independent professionals. Data analysis reports are sent to insurers for validation and verification. Finally, records are consolidated, and a report is generated17. Information is available for public consultation through an Open Database Connectivity (ODBC).
For the present study, we reviewed data collected between January 2015 and December 2019. All registered patients diagnosed with renal malformations (Q600, Q601, Q602, Q603, Q604, Q605, Q610, Q611, Q612, Q613, Q614, Q615, Q618, Q619, Q620, Q621, Q622, Q623, Q624, Q625, Q626, Q627, Q628, Q630, Q631, Q632, Q633, Q638, Q639), male genital malformations (Q530, Q531, Q532, Q539, Q540, Q541, Q542, Q543, Q544, Q548, Q549, Q550, Q551, Q552, Q553, Q554, Q555, Q556), and exstrophy-epispadias complex (Q640, Q641), according to the International Statistical Classification of Diseases and Health Problems 10th revision18 were included for analysis. No identifying variables were collected. We applied the diagnostic filters “confirmed new cases” and “confirmed repeated cases.” Diagnostic impression and unspecified cases were excluded. With the purpose of quantifying the number of patients with these diagnoses, the function “people attended” was used, which includes each patient only once even if the patient was attended multiple times during the time of the study.
The National Administrative Department of Statistics (Departamento Administrativo Nacional de Estadistica-DANE, by its Spanish acronym) is the entity responsible for collecting, processing, analyzing official statistics in Colombia19. The Vital Statistics Subsystem collects and processes information about all births and deaths that occur in the country. Live birth and death certificates are filled out by doctors, nurses, or authorized health personnel who attended the event in the institutions providing health services throughout the country. Live births are defined as a product of gestation after the expulsion or removal from of the mother’s body, regardless of the duration of the pregnancy. They must be able to breathe or give any other sign of life, such as heartbeat or umbilical cord pulsation20. For the present study, we included all registered live births during 2015-2019 in each department of the country and discriminated by gender.
Geographical variables (latitude and longitude) of each department were extracted using the DANE geoportal. This tool collects the information of georeferenced data and allows access to geographic limits and official maps of the Colombian territory.
All methods were performed in accordance with the principles established in the Declaration of Helsinki. This study is classified as a study without risk, according to resolution 8430-1993. It uses retrospective documentary research techniques with no intervention or intentional modification of the variables.
Statistical analysis
Cluster detection was performed using SaTScan v9.621 for macOS (Satscan, 2018) to identify clusters with either high or low rates of medical assessments over time. A spatial-temporal statistical analysis using a Poisson probability model was conducted for each diagnostic group. In the case file, we included all people with the specified diagnoses and in the population file, the live births were added. Latitude and longitude coordinates were entered for each department. The study period started on January 01, 2015, and ended on December 31, 2019. Each analysis was run using a time aggregation of 1 year of length and the option scan for areas with high or low rates was selected. The results obtained were visualized in a map from Google Earth.
Results
Between January 2015 and December 2019, a total of 26,726 patients with renal malformations, 19,149 patients with genital malformations, and 494 patients with exstrophy-epispadias complex in the outpatient clinic were assessed in Colombian territory.
In the case of renal malformations, space-time analysis identified one cluster with a high provided health-care concentration seen in the center of the country (Risaralda, Caldas, Quindío, Antioquia, Tolima, Valle del Cauca, Cundinamarca, Bogotá, D.C., and Huila) (Fig. 1 and Table 1). Meanwhile northern regions close to the coast (Bolívar, Sucre, Magdalena, Córdoba, Cesar, La Guajira, and Norte de Santander) were identified as clusters with low rates of provided care. Areas with the highest concentration of cases are shown in red. Meanwhile, clusters in blue show those municipalities below the average value assessed for the years analyzed.
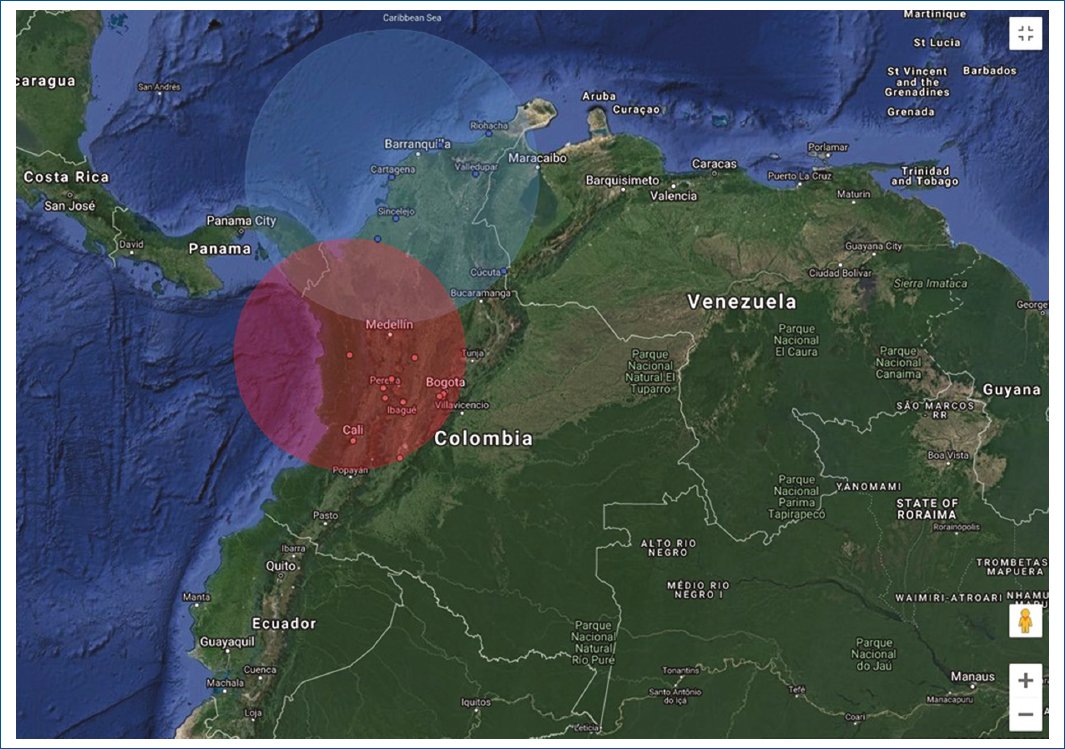
Figure 1. Spatial-temporal analysis of renal malformations.
Table 1. Identified clusters with an increase or reduction of cases assessed of renal malformations in Colombia during 2015-2019
| Cluster | Departments | Coordinates/radius | Detected cases | Population | Annual cases/100000 |
|---|---|---|---|---|---|
| 1 (Red) | Chocó, Risaralda, Caldas, Quindío, Antioquia, Tolima, Valle del Cauca, Cundinamarca, Bogotá, D.C., Huila | (5.692195 N, 76.625969 W)/340.81 km | 25,254 | 336,348 | 4,076.4 |
| 2 (Blue) | Bolívar, Sucre, Magdalena, Córdoba, Cesar, La Guajira, Norte de Santander | (10.384986 N, 75.496431 W)/428.24 km | 1,472 | 161,152 | 473.6 |
The genital malformations spatial scan statistical analysis (Fig. 2 and Table 2), which had a similar geographic distribution to the observed renal malformations, identified one cluster during the stu!dy time.
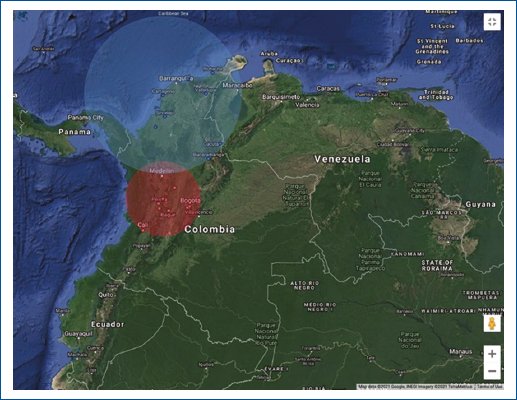
Figure 2. Spatial-temporal analysis of male genital malformations.
Table 2. Identified clusters of cases assessed of male genital malformations in Colombia during 2015-2019
| Cluster | Departments | Coordinates/radius | Detected cases | Population | Annual cases/100000 |
|---|---|---|---|---|---|
| 1 (Red) | Caldas, Risaralda, Quindío, Tolima, Chocó, Cundinamarca, Bogotá, D.C., Antioquia | (5.057688 N, 75.491049 W)/169.70 km | 18,371 | 129,590 | 7,387.6 |
| 2 (Blue) | Cesar, La Guajira, Magdalena, Atlántico, Bolívar, Sucre, Norte de Santander, Córdoba, Santander, Arauca | (10.460481 N, 73.259389 W)/466.36 km | 5,340 | 122,133 | 2,207.8 |
For cases with exstrophy-epispadias complex, a total of two clusters were identified (Fig. 3 and Table 3). Regions in the north had lower registered assessments compared to the center of the country.
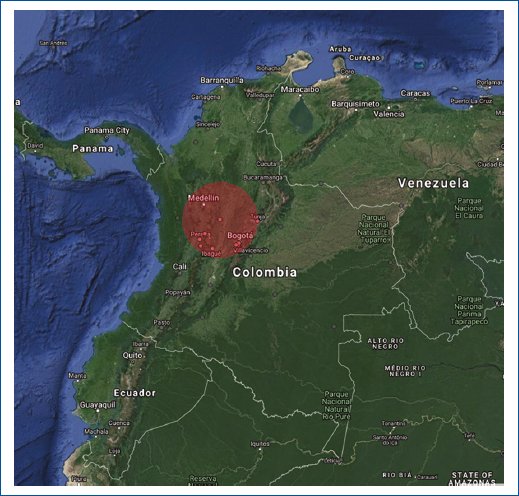
Figure 3. Spatial-temporal analysis of Exstrophy-epispadias complex.
Table 3. Identified clusters with an increase or reduction of cases assessed with a diagnosis of Exstrophy-epispadias complex in Colombia during 2015-2019
| Cluster | Departments | Coordinates/radius | Detected cases | Population | Annual cases/100000 |
|---|---|---|---|---|---|
| 1 (Red) | Caldas, Risaralda, Quindio, Tolima, Antioquia, Cundinamarca, Bogotá, D.C., Valle del Cauca | (5.057688 N, 75.491049 W)/215.38 km | 153 | 298,245 | 26.8 |
Discussion
Health-care distribution demonstrates a high cluster concentration of patients with renal malformations, male genital malformations, and exstrophy-epispadias complex in highly dense urban areas mostly distributed in the center of the country. Other major cities do not show high-concentration clusters. These results highlight unequitable distribution in the care of patients with congenital genitourinary malformations. Distance between home and hospitals, difficulty in obtaining medical care, and type of health-care provider might influence these results22. This has implications in patient prognosis, which in turn produces an increased cost to the health system. The identification of genitourinary malformation clusters can be used to optimize healthcare access23.
Bladder exstrophy-epispadias complex is a severe abdominal midline malformation in which reconstructive procedures significantly affect patients’ long-term quality of life24,25. Increased risk of urinary incontinence and the need for multiple reinterventions are some of the consequences of failed primary bladder closure26. It has been demonstrated that subspecialty surgical training has a positive influence on successful results27. In a United Kingdom study, Gupta et al.25 describe that the centralization of these patients has resulted in experienced multidisciplinary teams, follow-up and psychological support. These, in turn, improved overall outcomes for patients. Our results suggest that patients living in remote areas of the country are less likely to receive specialized care in a timely manner, which can affect their prognosis.
On the other hand, kidney congenital anomalies represent a significant cause of chronic kidney disease in children and young adults2,28. Previous studies have demonstrated that certain socioeconomic factors are related to ESRD due to inequitable access to health-care resources, including delayed referral29,30. Our study does not provide information about the percentage of patients who develop chronic kidney failure. However, the areas of the country where we do not find clusters with high rates of medical attention are those with the greatest economic limitations. These patients are probably underrepresented. A study by Sanna-Cherchi et al.31 followed a cohort of 312 children with renal and urinary tract anomalies until the age of 30 years. All patients with a diagnosis of posterior urethral valves had at least one surgical treatment, 35% of the children with solitary kidney and 22% of children with vesicoureteral reflux had ureteral reimplantation and all patients had been treated with prophylaxis for urinary tract infection for at least 1 year. This emphasizes the importance of an appropriate diagnosis and follow-up.
This is the first study to examine the spatial clusters of medical attention of genitourinary malformations in Colombia. Clustered areas need to be identified to facilitate the assessment of access to health care and design effective health interventions. In most cities, there are no subspecialists; therefore, we cannot know about factors such as the referral process and the waiting times. There are certain limitations to our study. Underreporting cases, methods of registration in certain areas, and differences in clinical practices or diagnostic techniques could have affected these results. Based on our findings, some recommendations can be proposed. It is necessary to collect more detailed information including waiting time for the first specialized consultation as well as construct a system of tracking and reference of these patients.
Conclusion
Patients with genitourinary malformations have inequitable access to specialized health care which can delay surgical management and impact long-term outcomes that increase disability. There is a greater concentration of cases assessed in the center of the country, where most of pediatric urologists, technology and hospitals are available.
Funding
The authors declare that they have not received funding.
Conflicts of interest
The authors declare no conflicts of interest.
Declarations
Availability of data and materials
The datasets generated during and/or analyzed during the current study are available to all public and were taken from Colombian government datasets. Information of RIPS is available for public consultation through an ODBC in Microsoft excel. Information about all registered live births during 2015-2019 in each department of the country are available at https://www.dane.gov.co/index.php/estadisticas-por-tema/salud/nacimientos-y-defunciones/nacimientos
Ethical disclosures
Protection of humans and animals. The authors declare that no experiments on humans or animals have been performed for this research.
Confidentiality of data. The authors declare that no patient data appear in this article. In addition, the authors have acknowledged and followed the recommendations according to the SAGER guidelines depending on the type and nature of the study.
Right to privacy and informed consent. The authors declare that no patient data appear in this article.
Use of artificial intelligence to generate texts. The authors declare that they have not used any type of generative artificial intelligence in the writing of this manuscript or for the creation of figures, graphs, tables, or their corresponding captions or legends.
