Introduction
Premature ejaculation (PE) is one of the most common male sexual dysfunctions, affecting approximately one-third of men worldwide1,2. It leads to distress in both the man and his sexual partner and has negative effects on quality of life3. According to the International Society of Sexual Medicine (ISSM), PE is characterized by ejaculation that occurs within 1 min after vaginal penetration4. However, the exact pathophysiology of PE remains unclear. Some studies have suggested that PE may result from a combination of psychogenic and biological factors, including penile hypersensitivity, anxiety, dysfunction of 5-HT receptors, and genetic predisposition5,6. However, most of them rarely seek for treatment7. PE treatment often involves a combination of pharmacotherapy and psychotherapy to address sexual abilities and psychological concerns.
Male circumcision is one of the most common surgical procedures worldwide, with nearly one-third of men aged 15 years or older undergoing circumcision for medical, religious, or cultural reasons8,9. Although circumcision has been associated with a reduced risk of urinary tract infections (UTIs), its role in penile sensitivity, specifically in the context of PE, remains a subject of controversy10. Some studies have reported an increase in penile sensitivity, while others have observed a reduction11. Morris and Krieger12 demonstrated that, in 2013, there was no correlation between circumcision and penile sensitivity, erectile dysfunction, PE, nor ejaculation time. Gao and Zhang13 found that the status of circumcision does not have a significant impact on intravaginal ejaculation latency time (IELT). Furthermore, it exerts control over ejaculation and satisfaction with sexual intercourse, as well as less severity in PE. One of the factors contributing to these conflicting findings is the impact of penile length (PL), penile skin length (PSL), and mainly mucosal cuff length (MCL) following this surgical procedure on PE. This study aims to provide a comprehensive review of the relationship between PL, PSL, and MCL, after circumcision and PE, a prevalent sexual dysfunction worldwide.
Materials and methods
This systematic review followed Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and the Cochrane handbook for systematic reviews of interventions. Our full protocol was registered in PROSPERO (registration number CRD42023429769). We defined our research question using the population, intervention, comparison, and outcome framework to guide the selection of studies. Population (P): Circumcised men. Intervention (I): Longer MCL comparison (C): Shorter MCL. Outcome (O): IELT or the PE diagnostic tool (PEDT) as measures of PE.
Eligibility criteria
This research primarily analyzed observational studies (case–control, cross-sectional). Three authors screened titles and abstracts to identify articles meeting the following inclusion criteria: (1) involvement of circumcised men; and (2) reporting of MCL measurement. The primary outcome measured was the IELT score. Exclusion criteria encompassed review articles, non-human studies, irrelevant articles, and duplicates.
Search strategy and study selection
Three authors conducted a keyword search on May 24, 2023, to identify relevant articles available in multiple databases (PubMed, ScienceDirect, Cochrane Library, ProQuest, Scopus, Web of Science, and Google Scholar). The keywords used are further elaborated in Table S1 in the supplementary material. A manual search was also performed on medRxiv to retrieve relevant articles meeting the specified criteria, while minimizing publication bias. Additional information regarding the search strategy is provided in the supplementary material. Titles and abstracts were individually screened to identify articles meeting the criteria and further assessed based on full articles. Any disagreements between the authors were resolved through discussions to reach a consensus.
Data extraction
Relevant data were independently extracted following a structured format that included information such as the first author, year of publication, research design, country of origin, sample size, mean age of patients, and crude values of MCL, PL, PSL, and IELT.
Quality assessment
The methodological quality of each study was assessed independently by two authors using the Newcastle–Ottawa scale (NOS) for case–control studies and an adapted NOS for cross-sectional studies. Studies were classified as “poor quality,” “fair quality,” or “good quality.”
Statistical analysis
Analyses were performed for both adjusted and unadjusted estimates; however, adjusted estimates were primarily utilized for reporting and interpretation of results whenever available. The mean and standard deviation (SD) for each penile measurement were pooled into forest plots. The random effects model was used to pool effect sizes to account for between-study heterogeneity; p < 0.05 was considered statistically significant. To control for the uncertainty in our estimate of the between-study heterogeneity, we used Knapp-Hartung adjustments to calculate the confidence interval around the pooled effect.
Whenever available, subgroup analyses were carried out for studies with (1) low risk of bias, (2) self-reported IELT, and (3) penis measured when erect. On the other hand, sensitivity analyses were conducted by leave-one-out analysis and the exclusion of studies with high-risk of bias. Meta-regression analyses were carried out for (1) year of publication; (2) sample size; and (3) mean age whenever possible. Meta-analysis was conducted with R ver. 4.3.0 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study selection
The initial search yielded a total of 78 records, from which 19 records were excluded due to duplication. After a rigorous screening process involving the assessment of titles and abstracts, nine potentially eligible articles were identified for further review. All articles were successfully retrieved. Following a comprehensive full-text assessment, eight studies met the criteria for inclusion in this systematic review, and seven studies were further analyzed for meta-analysis. A summary of the study selection process is depicted in the PRISMA flow chart depicted in figure 1.
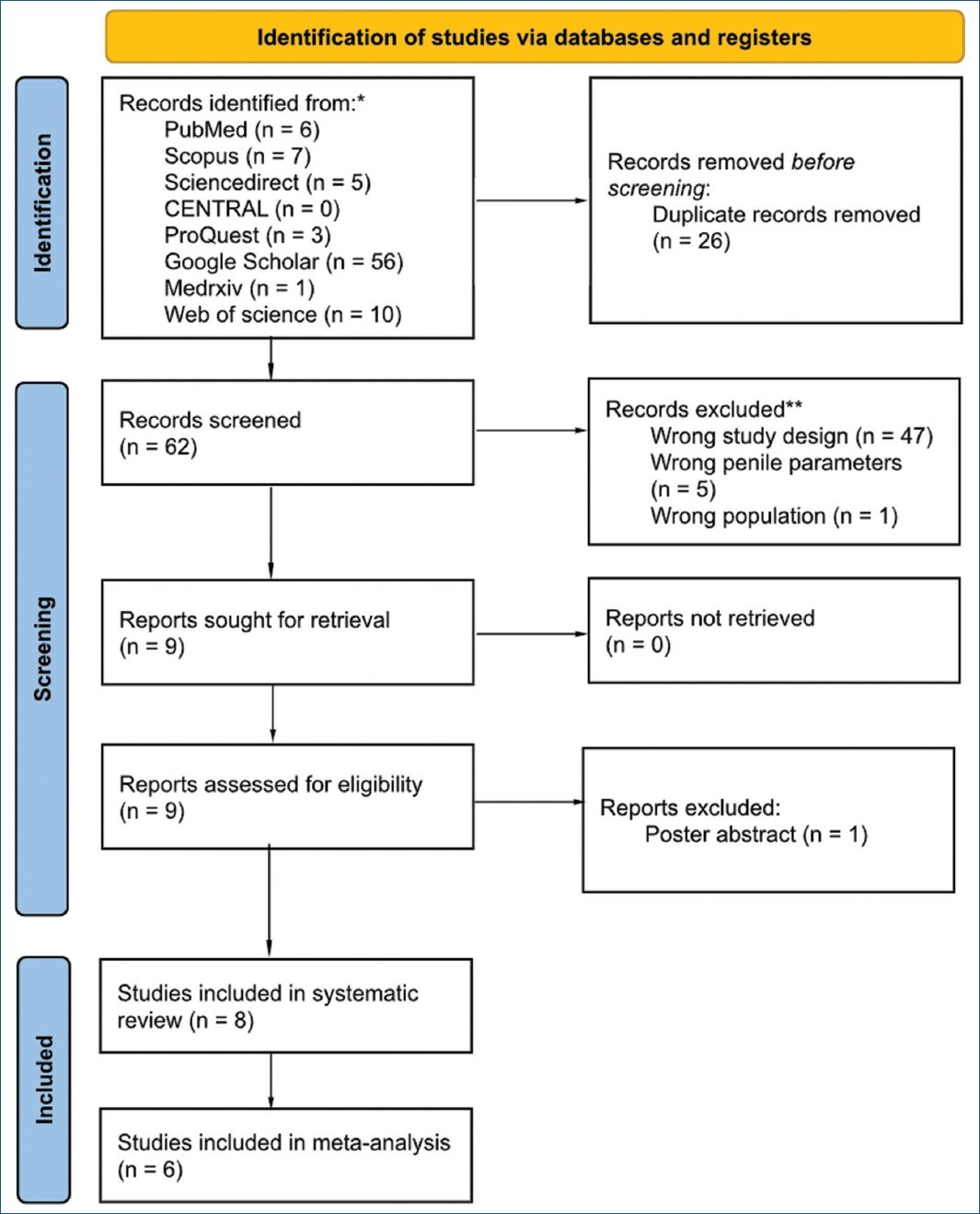
Figure 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses flowchart of the study selection process.
Quality assessment of included studies
Six of the studies included in this analysis were case–control studies, and two were cross-sectional studies. Each study was then evaluated using the NOS for case–control study and adapted NOS for cross-sectional study, respectively. A single reviewer conducted the evaluation according to the NOS guidelines, assigning an overall star rating based on specific criteria. Among the eight assessed studies, all studies were rated as good quality (Table 1)14–21.
Table 1. Risk of bias of included studies assessed with NOS tool
| NOS for case–control study | |||||
|---|---|---|---|---|---|
| No | Study | Selection | Comparability | Exposure | Overall star rating |
| 1 | Ates et al.; 2024 | **** | * | **** | Good quality |
| 2 | Gooran et al.; 2016 | *** | * | **** | Good quality |
| 3 | Hosseini et al.; 2008 | *** | ** | **** | Good quality |
| 4 | Tarhan et al.; 2013 | **** | * | **** | Good quality |
| 5 | Telli et al.; 2014 | *** | * | **** | Good quality |
| 6 | Yuruk et al.; 2016 | *** | * | **** | Good quality |
| NOS for cross-sectional study | |||||
| No | Study | Selection | Comparability | Outcome | Overall star rating |
| 1 | Bodakçi et al.; 2013 | ***** | * | ** | Good quality |
| 2 | Ongun et al.; 2020 | ***** | * | ** | Good quality |
|
NOS: Newcastle–Ottawa scale. The asterisks (*) represent the points awarded to each study for meeting specific quality criteria. |
|||||
Study characteristics
A total of eight studies were identified, comprising 1451 circumcised male participants, with 657 participants experiencing PE and 594 healthy participants serving as the control group. All the included studies were conducted in Asian regions. Most of the studies were primarily done in Turkey, while two studies were from Iran (Table 2).
Table 2. Characteristics of included studies
| No | Authors; year | Location | Study design | Sample size | PE diagnosis | Penile measurement | IELT measurement | Age | |
|---|---|---|---|---|---|---|---|---|---|
| IELT | Other tools | ||||||||
| 1 | Ates et al.; 2024 | Turkey | Case-control | 140 | NR | PEDT>9 | Flaccid | Self-reported | LLPE: 37.5 (18-61) AqPE: 33 (21-62) Control: 31 (18-62) |
| 2 | Bodakçi et al.; 2013 | Turkey | Cross-sectional | 200 | NR | PEDT | Flaccid | Stopwatch recorded | 35.1 (8.5) |
| 3 | Gooran et al.; 2016 | Iran | Case-control | 380 | < 2 min | NR | Erect | Stopwatch recorded | PE: 33.41 (8.9) Control: 34.7 (10.35) |
| 4 | Hosseini et al.; 2008 | Iran | Case-control | 84 | < 1 min | NR | Flaccid | Stopwatch recorded | PE: 34.2 (10.0) Control: 36.5 (9.1) |
| 5 | Ongun et al.; 2020 | Turkey | Cross-sectional | 208 | < 1 min | NR | Flaccid | Self-reported | PE: 35.1 (7.8) Control: 34.6 (9.0) |
| 6 | Tarhan et al.; 2013 | Turkey | Case-control | 160 | NR | NR | Flaccid | Stopwatch recorded | PE: 38.5 (10.3) Control: 38.7 (7.07) |
| 7 | Telli et al.; 2014 | Turkey | Case-control | 180 | < 1 min | NR | Flaccid | Stopwatch recorded | 32.7 (10.4) |
| 8 | Yuruk et al; 2016 | Turkey | Case-control | 99 | NR | NR | Flaccid | Self-reported | PE: 35.8 (7.7) Control: 38.8 (13.4) |
|
PE: premature ejaculation; IELT: intravaginal ejaculation latency time; NR: not reported; LLPE: lifelong premature ejaculation; AqPE: a\cquired premature ejaculation. |
|||||||||
Numerical data are presented as mean (SD) or median (interquartile range). Not reported, IELT, PE, PEDT, lifelong PE, acquired PE.
Patients’ characteristics
In this systematic review, the mean age of the participants was variable, ranging from 31 to 38.8 years. The studies used different methods to diagnose PE. Four of the eight studies used IELT as their main measure, but with different time limits: one study used < 2 min as their cutoff point, while three studies used < 1 min. Two other studies used the PEDT to identify PE. Most studies measured penis size in the flaccid state, except for Gooran et al.14 who took measurements during erection. Table 214–21 presents a detailed breakdown of these results. In addition, one study categorized the MCL into dorsal and ventral measurements15. All participants experiencing PE were patients from outpatient clinics.
IELT
In four of the included studies, IELT was employed as the primary outcome for assessing PE. IELT was defined as the duration from the initiation of vaginal intromission to the occurrence of intravaginal ejaculation, and it was measured using a stopwatch by female partners22. Participants were categorized as having PE if their IELT was < 60 s. In addition, two studies utilized ISSM diagnostic criteria for PE, which incorporates IELT as one of its measurement parameters23.
In the study conducted by Telli et al.18, participants were categorized into three groups: men with PE who were circumcised by doctors, men with PE who were circumcised by others, and a control group. The IELT in the first and second groups was notably lower than that in the control group (36 ± 6 s and 30 ± 6 s vs. 258 ± 20 s, p = 0.72), respectively. Furthermore, there was no significant difference in IELT between the first and second groups, as shown in figure 2.
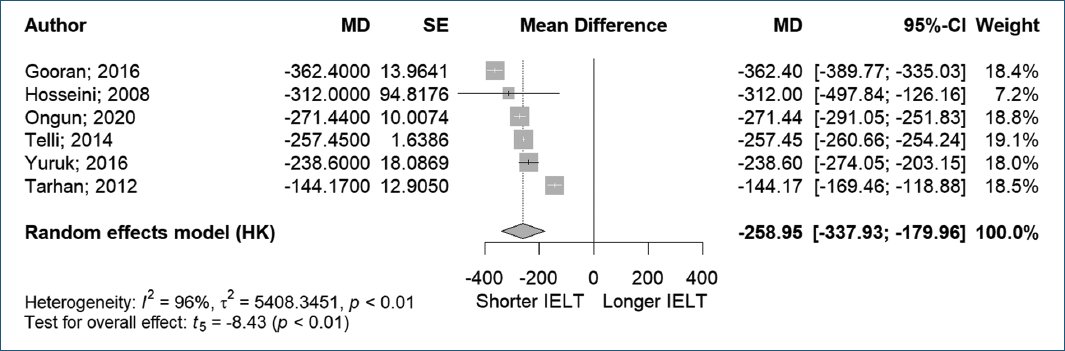
Figure 2. Intravaginal ejaculation latency time to premature ejaculation.
In this meta-analysis, six studies with a total of 587 patients with PE and 524 patients without PE were examined. Analysis using a random-effects model forest plot revealed that PE patients demonstrated significantly shorter IELTs compared to controls. However, the analysis showed high and statistically significant heterogeneity between studies (MD −258.95, 95% CI [−337.93, −179.96], p < 0.01, I2 = 96%).
In addition to IELT, Ongun et al.15 in 2020 also incorporated the PEDT in their study and observed significantly higher PEDT scores in the PE group compared to the control group (14.77 ± 2.56 vs. 4.47 ± 2.07, p < 0.001).
MCL
Five studies examined the MCL in both the PE and normal control groups. Gooran et al.14 found a significant relationship between MCL and PE (33.45 ± 9.99 mm vs. 30.86 ± 10.49 mm, p = 0.014). In contrast, Tarhan et al.19 found no significant difference in MCL between the PE and control groups (26.4 ± 11.2 mm vs. 26.1 ± 13.8 mm, p = 0.88), a result consistent with findings from Hosseini et al.20 (15.4 ± 4.8 mm vs. 14.7 ± 3.4 mm, p = 0.84) and Yuruk et al.21 (14.37 ± 4.92 mm vs. 12.88 ± 4.06 mm, p = 0.05). Similar findings were reported by Telli et al.18 where no significant difference in MCL was observed among the PE and control groups (11.7 ± 1.7 mm, 14.8 ± 3.1 mm, 12.8 ± 3.1 mm, p = 0.89).
Meanwhile, Ongun et al.15 divided the MCL measurement into dorsal and ventral MCL and found that the PE group had significantly longer dorsal and ventral MCL compared to the control group (16.87 ± 4.84 mm and 18.18 ± 5.35 mm vs. 13.10 ± 3.37 mm and 14.36 ± 3.50 mm, p < 0.001, respectively) (Fig. 3).
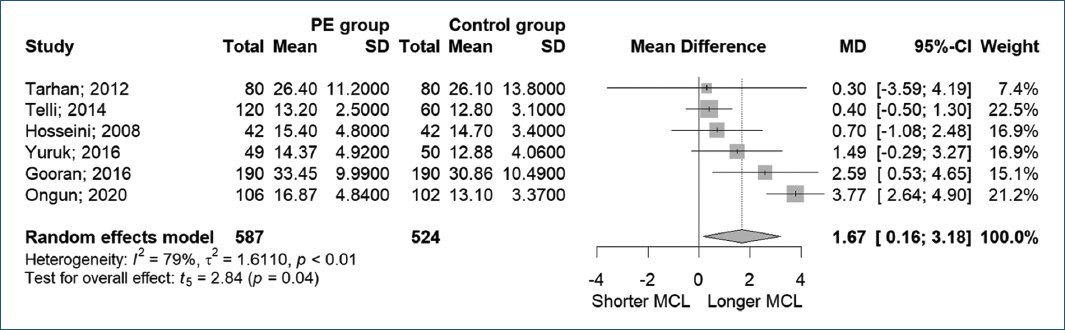
Figure 3. Mucosal cuff length to premature ejaculation.
In this meta-analysis, six studies with a total of 587 patients with PE and 524 patients without PE were examined. Using a random-effects model, the forest plot analysis indicated that patients with PE tended to have longer MCL compared to those without PE. However, substantial heterogeneity was observed among the included studies (MD = 1.67, 95% CI [0.16, 3.18], p = 0.04, I2 = 79%).
In addition, Yuruk et al.21 not only compared MCL to IELT but also measured the mucosal cuff to PL ratio (MCR) and reported a negative correlation with IELT (p = 0.0001). The PE group had a significantly longer MCR than those without PE (0.11 ± 0.03 cm vs. 0.09 ± 0.03 cm, p < 0.0001).
PL
Figure 4 showed five studies measured PL for comparison with IELT. Yuruk et al.21 reported that the PL of the PE group was significantly shorter than that of the control group (129 ± 26 mm vs. 146.1 ± 24 mm, p = 0.001). In contrast, Hosseini et al.20 and Ongun et al.,15 in 2020, stated that there was no significant correlation between PL and IELT. Telli et al.18 and Gooran et al.14 reported similar results, finding no difference in PL between PE and healthy participants (p = 0.69).
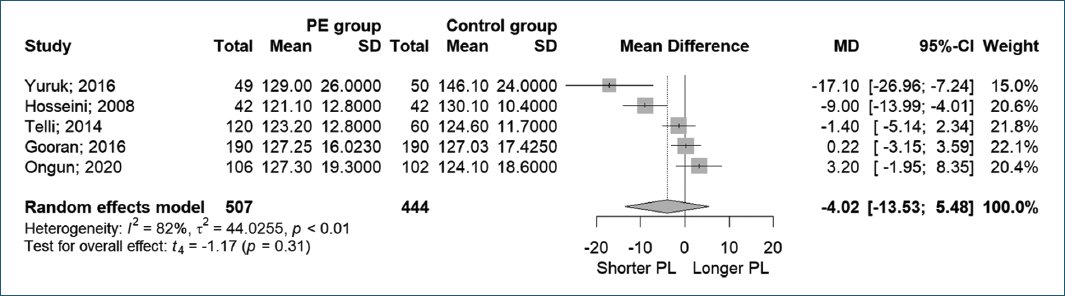
Figure 4. Penile length to premature ejaculation.
In this meta-analysis, the forest plot with a random-effects model demonstrated that PL had no significant effect on PE (MD −4.02, 95% CI [−13.53, 5.48], p < 0.01, I2 = 82%).
PSL
Two studies also measured PSL for comparison with IELT. Hosseini et al.20 and Telli et al.18 found no significant effect of PSL in patients with PE (80.8 ± 21 mm vs. 88.7 ± 12.2 mm, p = 0.99; 83.5 ± 15.5 mm vs. 84.2 ± 12.4 mm, p = 0.84), respectively (Fig. 5).
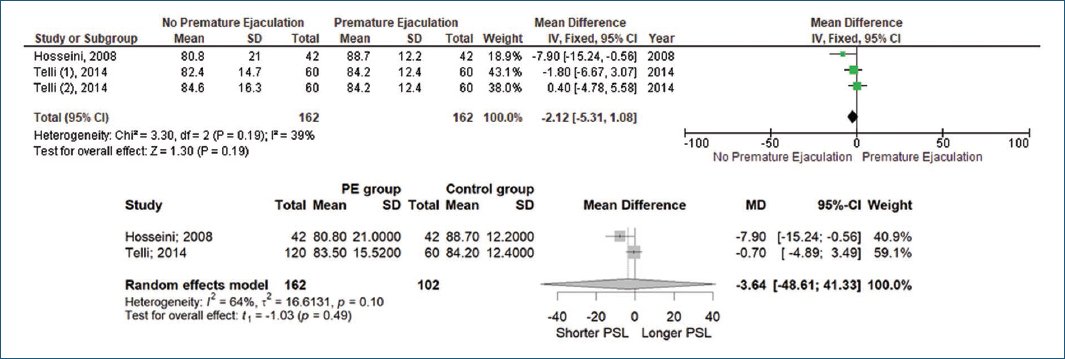
Figure 5. Penile skin length to premature ejaculation.
In this meta-analysis, three studies with a total of 162 patients with PE and 102 patients without PE were examined. The forest plot with a random-effects model demonstrated that PSL had no significant effect on PE (MD −3.64, 95% CI [−48.61, 41.33], p = 0.10, I2 = 64%).
Publication bias
Assessment of publication bias was initially planned using Egger’s test and funnel plot analysis. However, these analyses could not be performed due to the insufficient number of included studies (n < 10), as this would yield unreliable results24. To minimize potential publication bias, we implemented several strategies during the search process, including searching gray literature (conference proceedings, dissertations, and unpublished reports) and manually searching through the reference lists of included studies to identify additional relevant articles.
Discussion
PE is a prevalent concern, affecting nearly one third of men worldwide1,2. Yet, the pathophysiology of PE remains elusive. Studies suggest that PE may stem from a combination of psychogenic and biological factors, including penile hypersensitivity, endocrinopathy, and genetic predisposition5,6. Multinational reports by Waldinger et al.16 indicated a median IELT of 324 s (range 33-2,646 s).
Today, male circumcision is one of the most common surgical procedures worldwide. Approximately one-third of men aged 15 years and older undergo circumcision for medical, religious, or cultural reasons9. Although circumcision is known to prevent UTIs, its impact on penile sensibility remains a subject of debate10. Research on circumcision’s effect on IELT is inconclusive, with some studies reporting increased penile sensitivity and others suggesting decreased sensitivity11. In a study by Senkul et al.25 in 2004, IELT was significantly longer after circumcision (p = 0.02), indicating that circumcision may not be a risk factor for PE but could potentially serve as a preventive measure. Concerns also exist regarding post-circumcision MCL, PL, and PSL and its potential impact on PE later in life.
In this review, six studies discussed the relationship between MCL, PL, PSL, and PE. It is found that longer MCL was significantly associated with increased PE. This result is hypothesized to be related to the abundance of Meissner’s corpuscles in the prepuce compared to smooth mucosa, making it the most sensitive structure of the penis26. A larger mucosal cuff contains more sensory receptors, which could potentially lead to faster IELT and an increased likelihood of experiencing PE20. This theory may explain why the group with longer MCL had a shorter ejaculation time and higher penile sensitivity.
Conversely, some of previous studies concluded that MCL is not correlated with PE. Differences in circumcision techniques and penile sensitivity among participants may influence these findings. Another potential theory is that circumcision may reduce penile sensitivity by removing the preputial mucosa, which appears to be a vital component of the sensory mechanism in ejaculation27. Study by Telli et al.18 stated that men with a larger MCL indicate no correlation between post-circumcision MCL and PE. However, it was observed that the post-circumcision mucosa tended to be shorter when the procedure was performed by doctors. This theory is supported by Senkul et al.24 which stated that IELT was significantly longer after circumcision, suggesting an advantage rather than a complication. In contrast to the other studies mentioned, one study suggested that the longer the mucosal cuff to PL ratio (MCR), the shorter the IELT21.
In this meta-analysis, it is found that longer PL and PSL were not associated with increased PE. Both the studies by Hosseini et al.20 and Ongun et al.15 found no significant correlation between PL and IELT. Similarly, Telli et al.18 and Gooran et al.14 reported no significant difference in PL between individuals with PE and healthy participants. Two studies conducted by Hosseini et al.20 and Telli et al.18 measured PSL in relation to IELT for individuals with PE. Both studies found no significant correlation between PSL and PE.
To the best of our knowledge, this is the first systematic review investigating the effect of MCL, PL, and PSL on PE in circumcised men. However, this meta-analysis and systematic review has several limitations. First, these studies lack data on PE status or IELT before circumcision. Second, the number of included studies is limited, necessitating larger-scale studies with clinical trial designs to confirm the results. Third, some studies used self-estimated IELT rather than stopwatch measured IELT, which has a sensitivity and specificity of only 80%. Fourth, this study used unadjusted values for the pooled analysis, which may be subject to confounding. The findings should be interpreted with caution, considering the potential influence of unmeasured or unaccounted-for underlying influencing factors. Future research with larger and more diverse participant groups, along with histological analysis, is required to overcome the limitations of the current study.
Conclusion
This study found that there is a significant relationship between the length of penile mucosa and PE in circumcised patients. Patients with PE had significantly longer mucosal cuffs compared to those without. Moreover, no relationship was found between PL and PSL with PE in circumcised men. It could prove beneficial in the prevention of penile entrapment to avoid leaving an excess of dorsal and ventral mucosal tissue during circumcision. Furthermore, large-scale studies should be conducted to evaluate the positive and negative effects of circumcision in PE syndrome.
Acknowledgments
The authors would like to thank Intan Medika General Hospital and Faculty of Medicine Universitas Airlangga for the supports during this study.
Funding
The authors declare that this work was carried out with the authors’ own resources.
Conflicts of interest
The authors declare that they have no conflicts of interest.
Ethical considerations
Protection of humans and animals. The authors declare that no experiments involving humans or animals were conducted for this research.
Confidentiality, informed consent, and ethical approval. The study does not involve patient personal data nor requires ethical approval. The SAGER guidelines do not apply.
Declaration on the use of artificial intelligence. The authors declare that no generative artificial intelligence was used in the writing of this manuscript.
Supplementary data
Supplementary data are available at DOI: 10.24875/RUC.25000007. These data are provided by the corresponding author and published online for the benefit of the reader. The contents of supplementary data are the sole responsibility of the authors.
